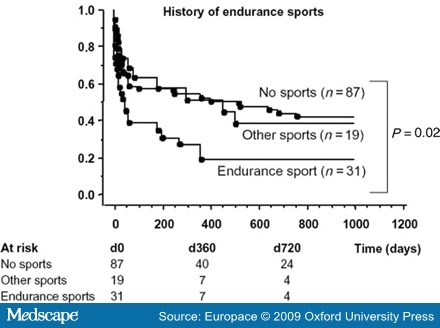
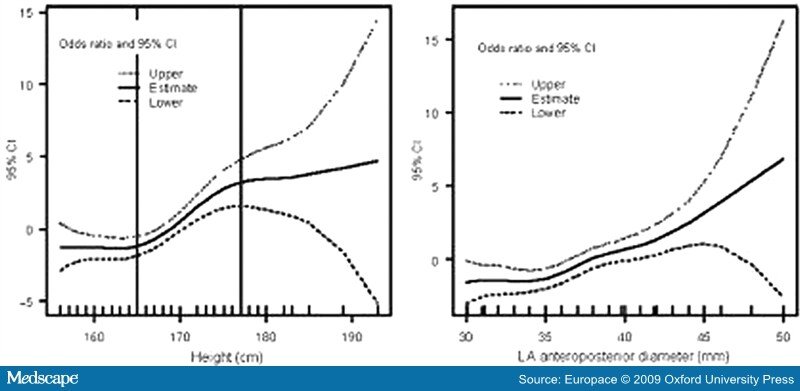
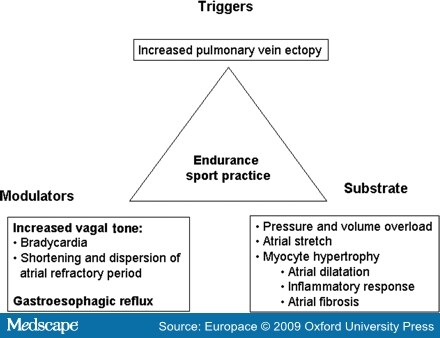
- Abusaada, K., Sharma, S.B., Jaladi, R. and Ezekowitz, M.D. (2004) Epidemiology and management of new-onset atrial fibrillation. Am J Manag Care 10: S50-57.
- Albert, C.M., Manson, J.E., Cook, N.R., Ajani, U.A., Gaziano, J.M. and Hennekens, C.H. (1999) Moderate alcohol consumption and the risk of sudden cardiac death among US male physicians. Circulation 100: 944-950.
- Anadon, M.J., Almendral, J., González, P., Zaballos, M., Delcan, J.L. and De Guevara, J.L. (1996) Alcohol concentration determines the type of atrial arrhythmia induced in a porcine model of acute alcoholic intoxication. Pacing Clin Electrophysiol 19: 1962-1967.
- Atkinson, L., Why, H., Ward, R.J., Peters, T.J. and Richardson, P.J. (1992) Antioxidant status in dilated cardiomyopathy and alcohol heart muscle disease. Alcohol Alcohol 28: 67-73.
- Benjamin, E.J., Levy, D., Vaziri, S.M., D'Agostino, R.B., Belanger, A.J. and Wolf, P.A. (1994) Independent risk factors for atrial fibrillation in a population-based cohort: The Framingham Heart Study. JAMA 271: 840-844.[Abstract]
- Buckingham, T.A., Kennedy, H.L., Goenjian, A.K., Vasilomanolakis, E.C., Shriver, K.K., Sprague, M.K. et al. (1985) Cardiac arrhythmias in a population admitted to an alcohol detoxification center. Am Heart J 110: 961-965.
- Camargo Jr, C.A., Hennekens, C.H., Gaziano, J.M., Glynn, R.J., Manson, J.E. and Stampfer, M.J. (1997) Prospective study of moderate alcohol consumption in US male physicians. Arch Intern Med 157: 79-85.
- Carpentier, R.G. and Gallardo-Carpentier, A. (1987) Acute and chronic effects of ethanol on sinoatrial electrophysiology in the rat heart. J Cardiovasc Pharmacol 10: 616-621.
- Chen, Y.C., Chen, S.A., Chen, Y.J., Tai, C.T., Chan, P. and Lin, C.I. (2004) Effect of ethanol on the electrophysiological characteristics of pulmonary vein cardiomyocytes. Eur J Pharmacol 483: 215-222.
- Chen, Y.J., Chen, S.A., Chen, Y.C., Yeh, H.I., Chan, P., Chang, M.S. et al. (2001) Effects of rapid pacing on the arrhythmogenic activity of single cardiomyocytes from pulmonary veins: implications in initiation of atrial fibrillation. Circulation 104: 2849-2854.
- Chen, Y.J., Chen, S.A., Chen, Y.C., Yeh, H.I., Chan, P., Chang, M.S. et al. (2002) Effects of thyroid hormone on the arrhythmogenic activity of pulmonary vein cardiomyocytes. J Am Coll Cardiol 39: 366-372.
- Cohen, E.J., Klatsky, A.L. and Armstrong, M.A. (1988) Alcohol use and supraventricular arrhythmia. Am J Cardiol 62: 971-973.
- Denison, H., Jern, S., Jagenburg, R., Wendestam, C. and Wallerstedt, S. (1994) Influence of increased adrenergic activity and magnesium depletion on cardiac rhythm in alcohol withdrawal. Br Heart J 72: 554-560.
- Di Castelnuovo, A., Rotondo, S., Iacoviello, L., Donati, M.B. and De Gaetano, G. (2002) Meta-analysis of wine and beer consumption in relation to vascular risk. Circulation 105: 2836-2844.
- Djousse, L., Levy, D., Benjamin, E.J., Blease, S.J., Russ, A., Larson, M.G. et al. (2004) Long-term alcohol consumption and the risk of atrial fibrillation in the Framingham study. Am J Cardiol 93: 710-713.
- Engel, T.R. and Luck, J.C. (1983) Effect of whiskey on atrial vulnerability and `holiday heart'. J Am Coll Cardiol 1: 816-818.[Abstract]
- Ettinger, P.O., Wu Chia, F., De La Cruz Jr, C., Weisse, A.B., Ahmed, S.S. and Reagan, T.J. (1978) Arrhythmias and the `Holiday Heart': alcohol-associated cardiac rhythm disorders. Am Heart J 95: 555-562.
- Fenelon, G. and Brugada, P. (1998) Unipolar waveforms and monophasic action potentials in the characterization of slow conduction in human atrial flutter. Pacing Clin Electrophysiol 21: 2580-2587.
- Fenelon, G., Balbao, C.E.B., Fernandes, R., Arfelli, A., Landim, P., Ayres, O. et al. (2007) Characterization of the acute cardiac electrophysiologic effects of ethanol in dogs. Alcohol Clin Exp Res 31: 1574-1580.
- Franz, M.R. (1991) Method and theory of monophasic action potential recording. Prog Cardiovasc Dis 33: 347-68.
- Friberg, J., Scharling, H., Gadsboll, N. and Jensen, G.B. (2003) Sex-specific increase in the prevalence of atrial fibrillation (The Copenhagen City Heart Study). Am J Cardiol 92: 1419-1423.
- Friedman, L.A. and Kimball, A.W. (1986) Coronary heart disease mortality and alcohol consumption in Framingham. Am J Epidemiol 124: 481-489.
- Frost, L. and Vestergaard, P. (2004) Alcohol and risk of atrial fibrillation or flutter - a cohort study. Arch Intern Med 164: 1993-1998.
- Goodking, M.J., Gerber Jr, N.H., Mellen, J.R. and Kostis, J.B. (1975) Altered intracardiac conduction after acute administration of ethanol in the dog. J Pharmacol Exp Ther 194: 633-638.
- Gould, L., Reddy, C.V., Becker, W., Oh, K.C. and Kim, S.G. (1978) Electrophysiologic properties of alcohol in man. J Electrocardiol 11: 219-226.
- Greenspon, A.J. and Schaal, S.F. (1983) The `holiday heart': electrophysiologic studies of alcohol effects in alcoholics. Ann Intern Med 98: 135-139.
- Gribaldo, R.G., Pomoni, G.S. and Sale, F. (1985) Arrhythmias and left function in chronic alcoholics with cirrhosis. Am J Cardiol 56: 825-827.
- Guarnieri, T. and Lakatta, E.G. (1990) Mechanism of myocardial contractile depression by clinical concentrations of ethanol. J Clin Invest 85: 1462-1467.
- Guize, L., Thomas, F., Bean, K., Benetos, A. and Pannier, B. (2007) Fibrillation atriale: prevalence, facteurs de risqué et mortalité dans une vaste population française suivie 15 ans. Bull Acad Natl Med 191: 791-803.
- Habuchi, Y., Furukawa, T., Tanaka, H., Lu, L.L., Morikawa, J. and Yoshimura, M. (1995) Ethanol inhibition of Ca2+ and Na+ currents in the guinea-pig heart. Eur J Pharmacol 292: 143-149.
- Horton, J.W. and White, J.D. (1996) Cardiac contractile and sarcoplasmic reticulum function after acute ethanol consumption. J Surg Res 64: 132-138.
- Institute for Health Policy, Brandeis University. (1993) Substance Abuse: The Nation's Number One Health Problem. Princeton, NJ: Robert Wood Johnson Foundation.
- Jain, A.K. and Carpentier, R.G. (1998) Cardiac electrophysiological actions and interactions of ethanol, cocaine, and the metabolite ethylcocaine. J Electrocardiol 31: 293-302.
- Koskinen, P., Kupari, M., Leinonon, H. and Luomanmaöki, K. (1987) Alcohol and new onset atrial fibrillation: a case - control study of a current series. Br Heart J 57: 468-473.
- Koskinen, P., Kupari, M. and Leinonen, H. (1990) Role of alcohol in recurrences of atrial fibrillation in persons less than 65 years of age. Am J Cardiol 66: 954-958.
- Kostis, J.B., Goodkind, J., Skvaza, H., Gerber Jr, N.H. and Kuo, P.T. (1977) Effect of alcohol on the atrial fibrillation threshold in dogs. Angiology J Vasc Dis 28: 583-587.
- Koul, P.B., Sussmane, J.B., Cunill-De Sautu, B. and Minarik, M. (2005) Atrial fibrillation associated with alcohol ingestion in adolescence: holiday heart in pediatrics. Pediatr Emerg Care 21: 38-39.
- Krahn, A.D., Manfreda, J., Tate, R.B., Mathewson, F.A.L. and Cuddy, T.E. (1995) The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med 98: 476-484.
- Kupari, M. and Koskinen, P. (1991) Time of onset of supraventricular tachyarrhythmia in relation to alcohol consumption. Am J Cardiol 67: 718-722.
- Lorsheyd, A., de Lange, D.W., Hijmering, M.L., Cramer, M.J. and van de Wiel, A. (2005) PR and QtC interval prolongation on the electrocardiogram after binge drinking in health individuals. Neth J Med 63: 59-63.
- Lowenstein, S.R., Gabow, P., Cramer, J., Oliva, P.B. and Ratner, K. (1983) The role of alcohol in new-onset atrial fibrillation. Arch Intern Med 143: 1882-1885.
- Madan, B.R. and Gupta, R.S. (1967) Effect of ethanol in experimental auricular and ventricular arrhythmias. Jpn J Pharmacol 17: 683-684.
- Maöki, T., Toivonen, L., Koskinen, P., Naöveri, H., Haörkonen, M. and Leinonen, H. (1998) Effect of ethanol drinking, hangover, and exercise on adrenergic activity and heart rate variability in patients with a history of alcohol-induced atrial fibrillation. Am J Cardiol 82: 317-322.
- Marcus, G.M., Smith, L.M., Whiteman, D., Tseng, Z.H., Badhwar, N., Lee, B.K. et al. (2008) Alcohol intake is significantly associated with atrial flutter in patients under 60 years of age and a shorter right atrial effective refractory period. Pacing Clin Electrophysiol 31: 266-272.
- Moe, G.K. and Abildskov, J.A. (1959) Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J 58: 59-70.
- Mukamal, K.J., Psaty, B.M., Rautaharju, P.M., Furberg, C.D., Kuller, L.H., Mittleman, M.A. et al. (2007) Alcohol consumption and risk and prognosis of atrial fibrillation among older adults: the Cardiovascular Health Study. Am Heart J 153: 260-266.
- Mukamal, K.J., Tolstrup, J.S., Friberg, J., Jensen, G. and Gronbaek, M. (2005) Alcohol consumption and risk of atrial fibrillation in men and women - the Copenhagen City Heart Study. Circulation 112: 1736-1742.
- Nattel, S. and Opie, L.H. (2006) Controversies in atrial fibrillation. Lancet 367: 262-272.
- Nguyen, T.N., Friedman, H.S. and Mokraoui, A.M. (1987) Effects of alcohol on experimental atrial fibrillation. Alcohol Clin Exp Res 11: 472-476.
- Patel, V.C., Why, H.J., Richardson, P.J. and Preedy, V.R. (1997) The effects of alcohol on the heart. Adverse Drug React Toxicol Rev 16: 15-43.
- Planas, F., Romero-Menor, C., Vasquez-Oliva, G., Poblet, T. and Navarro-López, F. (2006) Historia natural y factores de riesgo de recurrencia de la fibrilación auricular primaria (Registro FAP). Rev Esp Cardiol 59: 1106-1112.
- Preedy, V.R., Siddiq, T., Why, H. and Richardson, P.J. (1994) The deleterious effects of alcohol on the heart: involvement of protein turnover. Alcohol Alcohol 29: 141-147.
- Psaty, B.M., Manolio, T.A., Kuller, L.H., Kronmal, R.A., Cushman, M., Fried, L.P. et al. (1997) Incidence of and risk factors for atrial fibrillation in older adults. Circulation 96: 2455-2461.
- Rensma, P.L., Allessie, M.A., Lammers, W.J.E.P., Bonke, F.I.M. and Schalij, M.J. (1988) Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ Res 62: 395-410.
- Rich, E.C., Siebold, C. and Campion, B. (1985) Alcohol-related acute atrial fibrillation, a case-control study and review of 40 patients. Arch Intern Med 145: 830-833.
- Rosenqvist, M. (1998) Alcohol and cardiac arrhythmias. Alcohol Clin Exp Res 22: 318S-322S.
- Snoy, F.J., Harker, R.J., Thies, W. and Greenspan, K. (1980) Ethanol-induced electrophysiological alterations in canine Purkinje fibers. J Stud Alcohol 41: 1023-1030.
- Steinbigler, P., Haberl, R., Koönig, B. and Steinbeck, G. (2003) P-wave signal averaging identifies patients prone to alcohol-induced paroxysmal atrial fibrillation. Am J Cardiol 91: 491-494.
- Stewart, S., Hart, C.L., Hole, D.J. and McMurray, J.J. (2001) Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart 86: 516-521.
- Thornton, J.R. (1984) Atrial fibrillation in healthy non-alcoholic people after an alcoholic binge. Lancet 2: 1013-1015.
- US Department of Health and Human Service. ( 1995) The Physician's Guide to Helping Patients with Alcohol Problems. Washington, DC: US Department of Health and Human Services, NIH Publication 95-3769.
- US Department of Health and Human Service ( 1996) Preliminary Estimates from 1995. National Household Survey and Drug Abuse. Advance Report 18., Rockville MD: National Institute on Drug Abuse.
- Uyarel, H.O.C., Karabulut, A., Okmen, E. and Cam, N. (2005) Acute alcohol intake and P-wave dispersion in health men. Anadolu Kardiyol Derg 5: 289-293.
- Vary, T.C., Deiter, G. and Goodman, A.S. (2005) Acute alcohol intoxication enhances myocardial eIF4G phosphorylation despite reducing mTOR signaling. Am J Physiol Heart Circ Physiol 288: H121-128.
- West, T.C. and Landa, J.F. (1962) Minimal mass required for induction of a sustained arrhythmia in isolated atrial segments. Am J Physiol 202: 232-236.
- Whyte, G., Stephens, N., Sharma, S., Shave, R., Budgett, R. and McKenna, W.J. (2004) Spontaneous atrial fibrillation in a freestyle skier. Br J Sports Med 38: 230-232.
- Wiener, N. and Rosenblueth, A. (1946) The mathematical formulation of the problem of conduction of impulses in a network of connected excitable elements, specifically in cardiac muscle. Arch Inst Cardiol Mex 16: 205-265.
- Wilhelmsen, L., Rosengren, A. and Lappas, G. (2001) Hospitalizations for atrial fibrillation in the general male population: morbidity and risk factors. J Intern Med 250: 382-389.
- Williams, E.S., Mirro, M.J. and Bailey, J.C. (1980) Electrophysiological effects of ethanol, acetaldehyde, and acetate on cardiac tissues from dog and guinea pig. Circ Res 47: 473-478.
- Wolf, P.A., Abbott, R.D. and Kannel, W.B. (1987) Atrial fibrillation: a major contributor to stroke in the elderly: The Framingham Study. Arch Intern Med 147: 1561-1564.
- Wolf, P.A., Abbott, R.D. and Kannel, W.B. (1991) Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 22: 983-988.
- Wolf, P.A., Benjamin, E.J., Belanger, A.J., Kannel, W.B., Levy, D. and D'Agostino, R.B. (1996) Secular trends in the prevalence of atrial fibrillation: The Framingham Study. Am Heart J 131: 790-795.
- Wong, M. (1973) Depression of cardiac performance by ethanol unmasked during autonomic blockade. Am Heart J 86: 508-515.
- World Health Organization (WHO) (1999) Global Status Report on Alcohol: 1999. Geneva: WHO. Available at: http://www.who.int/substance_abuse/publications/en/GlobalAlcohol_overview.pdf
|